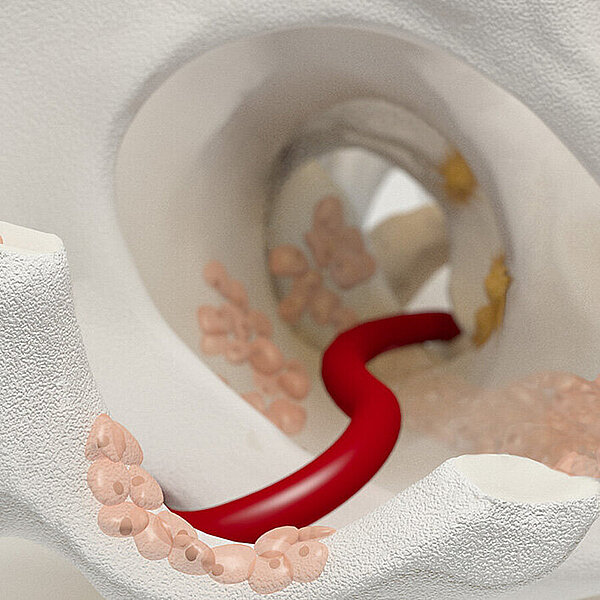
Chondro-Gide®

Chondro-Gide® è una membrana bilayer di collagene I/III sviluppata specificamente per la rigenerazione della cartilagine. Realizzata a partire da collagene suino altamente raffinato, è prodotta in Svizzera secondo un rigoroso sistema di garanzia della qualità per assicurarne la sicurezza e la qualità.1
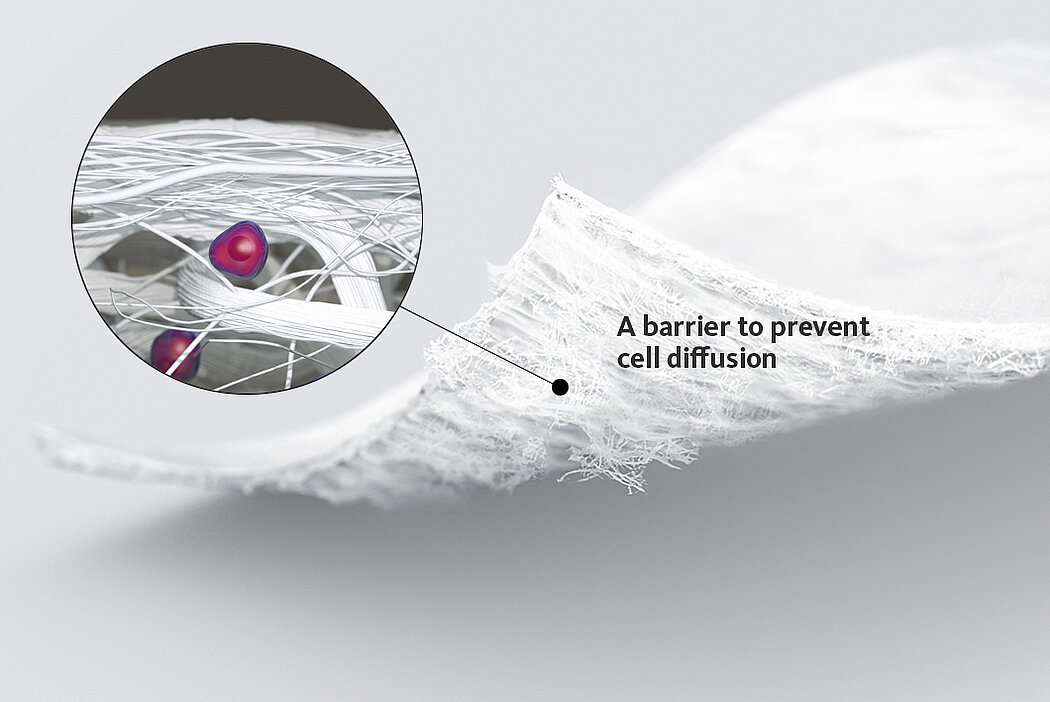
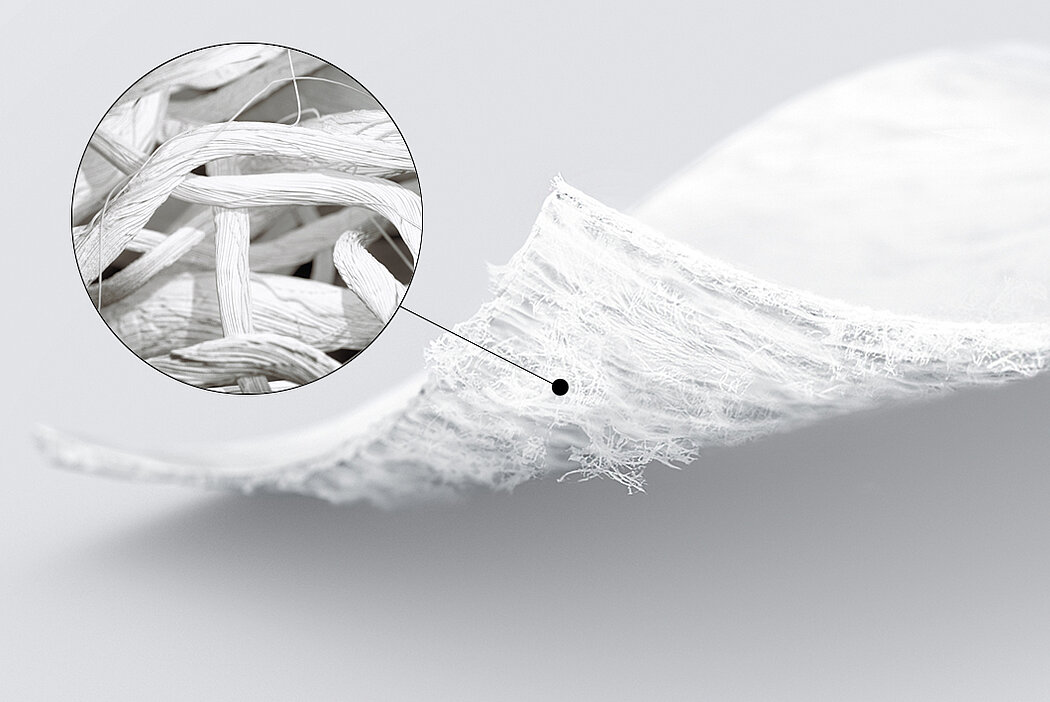
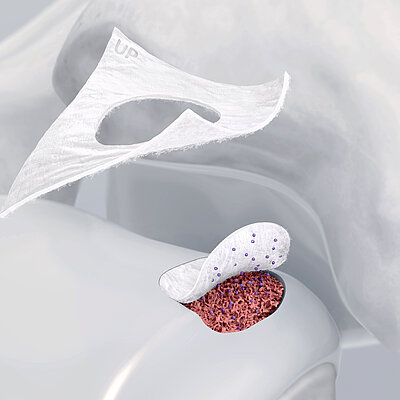
Chondro-Gide® works to leverage the body’s own healing potential to regenerate human cartilage. With a smooth, compact top layer, and a rough, porous bottom layer, its structure provides the conditions for cartilage regeneration.
Bibliografia:
- Geistlich Pharma AG data on file (Bench test)
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- VOLZ, M., et al. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop, Apr 2017, 41(4), 797-804. (Clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 63(5), 616-626. (Clinical study)
- GOMOLL, A.H., Surgical Management of Articular Cartilage Defects of the Knee. J Bone Joint Surg Am. 2010;92:2470-90 (Clinical study)
- MUMME, M., Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet, 2016, 388 (10055) 1985-1994. (Clinical study)
- FULCO, I., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet, Jul 26 2014, 84(9940), 337-346. (Clinical study)
- STEINWACHS, M.R., New Technique for Cell-Seeded Collagen Matrix-Supported Autologous Chondrocyte Transplantation, Arthroscopy: The Journal of Arthroscopic & Related Surgery Volume 25, Issue 2, February 2009, Pages 208-211 (Technical note)
- STEINWACHS, M. R., et al., "Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee." Cartilage 2019: 1947603519870846. (Review of clinical studies)
- CIEMNIEWSKA-GORZELA, K., et al.,Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: Clinical Effectiveness and Survivorship after a Minimum of 5 Years’Follow-Up. Cartilage. 2020 Jun 1 (Clinical study)
Vantaggi
Il successo di Chondro-Gide® è supportato da oltre 10 anni di dati clinici2. Grazie alla sua struttura bilayer appositamente studiata, Chondro-Gide® fornisce un ambiente protettivo che favorisce la crescita di nuova cartilagine.3,4
- Membrana bilayer di Collagene I/III bio-derivata1
- Biocompatibile e a riassorbimento naturale1
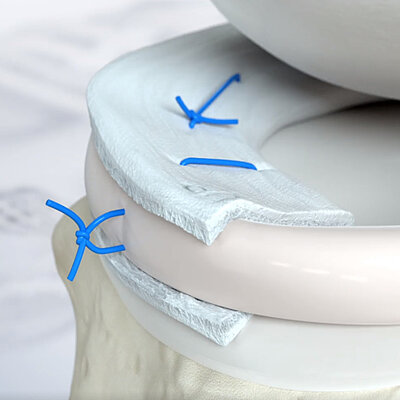
- Può essere incollata o suturata in posizione4
- Compatibile con una serie di tecniche di rigenerazione tissutale5
- Procedura in un'unica fase1
- Facile da maneggiare: elastico e resistente agli strappi1
- Pronto per l'uso a scaffale1
Come funziona Chondro-Gide®
Bibliografia:
- Geistlich Pharma AG data on file (Bench test)
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- VOLZ, M., et al. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop, Apr 2017, 41(4), 797-804. (Clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 63(5), 616-626. (Clinical study)
- GOMOLL, A.H., Surgical Management of Articular Cartilage Defects of the Knee. J Bone Joint Surg Am. 2010;92:2470-90 (Clinical study)
- MUMME, M., Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet, 2016, 388 (10055) 1985-1994. (Clinical study)
- FULCO, I., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet, Jul 26 2014, 84(9940), 337-346. (Clinical study)
- STEINWACHS, M.R., New Technique for Cell-Seeded Collagen Matrix-Supported Autologous Chondrocyte Transplantation, Arthroscopy: The Journal of Arthroscopic & Related Surgery Volume 25, Issue 2, February 2009, Pages 208-211 (Technical note)
- STEINWACHS, M. R., et al., "Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee." Cartilage 2019: 1947603519870846. (Review of clinical studies)
- CIEMNIEWSKA-GORZELA, K., et al.,Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: Clinical Effectiveness and Survivorship after a Minimum of 5 Years’Follow-Up. Cartilage. 2020 Jun 1 (Clinical study)
Tecniche chirurgiche con Condro-Gide®
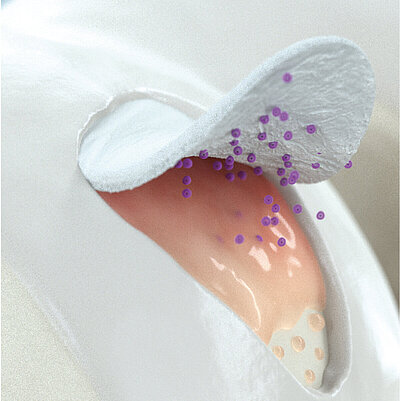
Chondro-Gide® come vettore
Nelle tecniche di impianto di condrociti autologhi, Chondro-Gide® funge da vettore. Fornisce una matrice 3D di supporto per l'adesione e la differenziazione cellulare9. Le cellule possono essere seminate sul lato ruvido della membrana bilayer, mentre il lato liscio impedisce la perdita di cellule durante il processo.
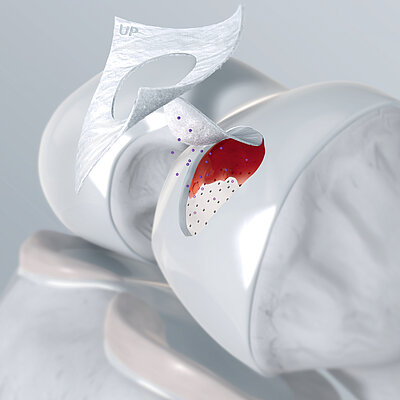
Chondro-Gide® come copertura
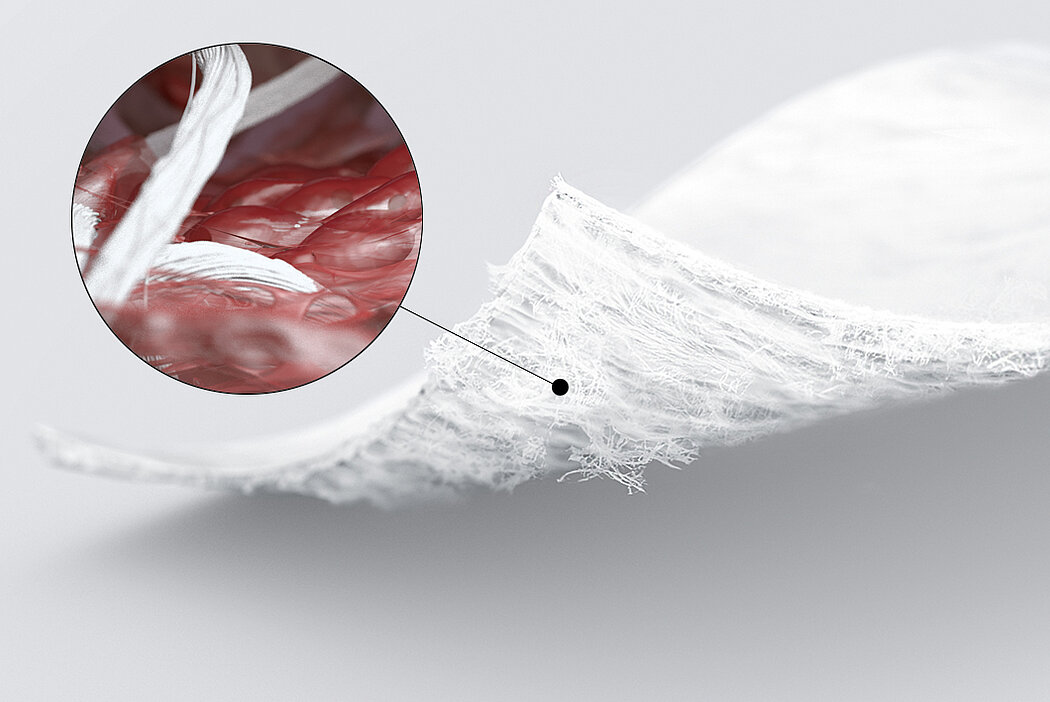
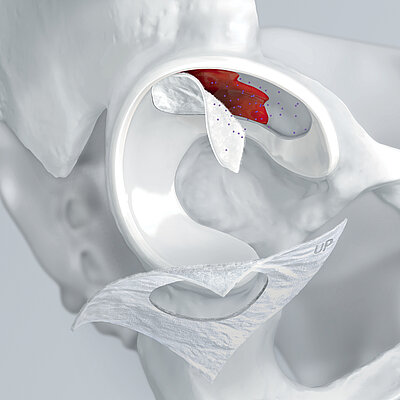
L'AMIC® (condrogenesi indotta da matrice autologa) è una tecnica in un unico passaggio, economica ed efficace per il trattamento dei difetti cartilaginei. In AMIC®, Chondro-Gide® ricopre il difetto e offre un ambiente protettivo per la differenziazione cellulare e la formazione di nuova cartilagine3,7,10. Per saperne di più su AMIC® qui
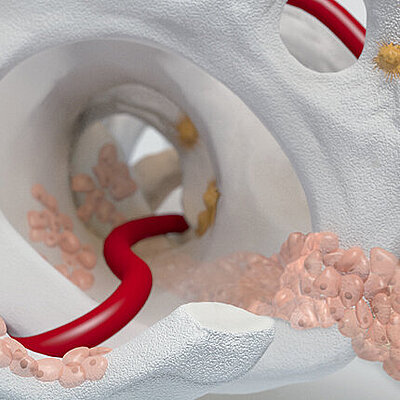
Condro-Gide® come impacco
L'AMMR® (Arthroscopic Matrix-Based Meniscus Repair) è una tecnica progettata per preservare il menisco. La membrana viene avvolta intorno al menisco danneggiato, con il lato liscio rivolto verso la cavità articolare11. In uno studio, lo spazio tra il menisco e la membrana avvolgente è stato aumentato con fattori biologici per migliorare il processo di riparazione.
Bibliografia:
- Geistlich Pharma AG data on file (Bench test)
- KAISER, N., et al. Clinical results 10 years after AMIC in the knee. Swiss Med Wkly, 2015, 145 (Suppl 210), 43S. (Clinical study)
- GILLE, J., et al. Cell-Laden and Cell-Free Matrix-Induced-Chondrogenesis versus Microfracture for the Treatment of Articular Cartilage Defects: A Histological and Biomechanical Study in Sheep. Cartilage OnlineFirst, January 7, 2010, doi:10.1177/1947603509358721 (Pre-clinical study)
- VOLZ, M., et al. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop, Apr 2017, 41(4), 797-804. (Clinical study)
- KRAMER, J., et al. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci, Mar 2006, 63(5), 616-626. (Clinical study)
- GOMOLL, A.H., Surgical Management of Articular Cartilage Defects of the Knee. J Bone Joint Surg Am. 2010;92:2470-90 (Clinical study)
- MUMME, M., Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet, 2016, 388 (10055) 1985-1994. (Clinical study)
- FULCO, I., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet, Jul 26 2014, 84(9940), 337-346. (Clinical study)
- STEINWACHS, M.R., New Technique for Cell-Seeded Collagen Matrix-Supported Autologous Chondrocyte Transplantation, Arthroscopy: The Journal of Arthroscopic & Related Surgery Volume 25, Issue 2, February 2009, Pages 208-211 (Technical note)
- STEINWACHS, M. R., et al., "Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee." Cartilage 2019: 1947603519870846. (Review of clinical studies)
- CIEMNIEWSKA-GORZELA, K., et al.,Meniscus Tears Treated with Collagen Matrix Wrapping and Bone Marrow Blood Injection: Clinical Effectiveness and Survivorship after a Minimum of 5 Years’Follow-Up. Cartilage. 2020 Jun 1 (Clinical study)